The standard product refers to a reference substance used for the quantitative analysis of traditional Chinese medicines, herbal preparations, and decoction pieces. Certified bacterial strains are procured by designated personnel from the Quality Assurance Department and sourced from the National Institute of Biological Sciences. Every time a request is made for registration, the name, quantity, purpose, and date must be recorded. These materials should be sealed and stored in a frost-free refrigerator, with test bacteria kept at a temperature between 3°C and 5°C.
 Standard titrants must be prepared and calibrated by the QC supervisor. After three months, they should be re-labeled, and complete records of preparation, calibration, and re-labeling must be maintained. If any abnormalities such as cloudiness, sedimentation, or discoloration occur during storage, immediate action should be taken.
â–³ Preparation Quality Requirements
For standard solutions that do not require calibration, analytically pure substances can be used. The water used in preparation must meet the standards outlined in the pharmacopoeia. Calibration standards should not use reference substances. To prevent moisture absorption, the material should be dried to a constant weight before calibration. According to pharmacopoeia guidelines, perchloric acid titrants should be prepared at room temperature. If the temperature difference between usage and calibration exceeds 4°C or goes beyond 10°C, the solution should be recalibrated or adjusted accordingly.
Standard titrants must be prepared and calibrated by the QC supervisor. After three months, they should be re-labeled, and complete records of preparation, calibration, and re-labeling must be maintained. If any abnormalities such as cloudiness, sedimentation, or discoloration occur during storage, immediate action should be taken.
â–³ Preparation Quality Requirements
For standard solutions that do not require calibration, analytically pure substances can be used. The water used in preparation must meet the standards outlined in the pharmacopoeia. Calibration standards should not use reference substances. To prevent moisture absorption, the material should be dried to a constant weight before calibration. According to pharmacopoeia guidelines, perchloric acid titrants should be prepared at room temperature. If the temperature difference between usage and calibration exceeds 4°C or goes beyond 10°C, the solution should be recalibrated or adjusted accordingly.
 â–³ Titration Calibration and Labeling
Before calibration, the titration solution must be shaken and left to stand for three days. The calibration method should follow the procedures specified in the pharmacopoeia. Typically, one person performs the calibration, while a second person conducts a parallel test. At least three parallel determinations should be carried out. The relative deviation of the results should not exceed 0.1%.
The relative deviation between the first and second test results should not exceed 0.15%. If this limit is exceeded, but the recalibration meets the requirements, the average of the two results should be used as the final titration value.
Qualified titrants and standard solutions must be clearly labeled with the product name, concentration, preparation date, calibration date, temperature, calibrator, preparer, and expiration date. Titrants should be relabeled periodically, and their shelf life is generally three months. Once expired, they should not be used.
Titrants and standard solutions should be stored according to the guidelines provided in the Chinese Pharmacopoeia. Solutions that are sensitive to light should be stored in brown bottles. The containers should be protected from moisture and contamination, and should be managed by designated personnel. Any standard solution or titrant that has passed its expiration date must not be issued by the custodian or used by the end-user.
â–³ Titration Calibration and Labeling
Before calibration, the titration solution must be shaken and left to stand for three days. The calibration method should follow the procedures specified in the pharmacopoeia. Typically, one person performs the calibration, while a second person conducts a parallel test. At least three parallel determinations should be carried out. The relative deviation of the results should not exceed 0.1%.
The relative deviation between the first and second test results should not exceed 0.15%. If this limit is exceeded, but the recalibration meets the requirements, the average of the two results should be used as the final titration value.
Qualified titrants and standard solutions must be clearly labeled with the product name, concentration, preparation date, calibration date, temperature, calibrator, preparer, and expiration date. Titrants should be relabeled periodically, and their shelf life is generally three months. Once expired, they should not be used.
Titrants and standard solutions should be stored according to the guidelines provided in the Chinese Pharmacopoeia. Solutions that are sensitive to light should be stored in brown bottles. The containers should be protected from moisture and contamination, and should be managed by designated personnel. Any standard solution or titrant that has passed its expiration date must not be issued by the custodian or used by the end-user.
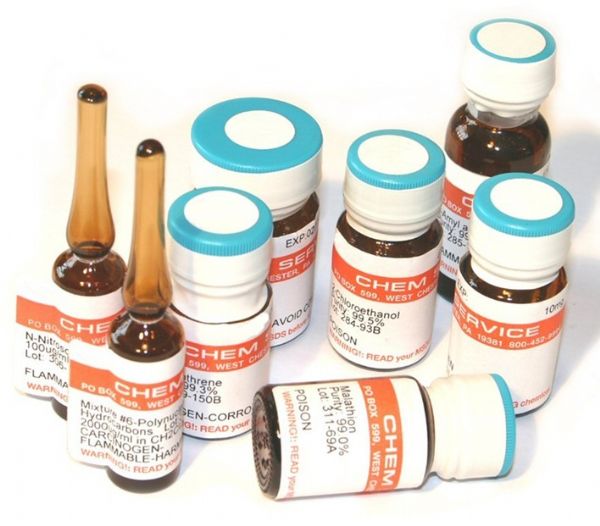
 Standard titrants must be prepared and calibrated by the QC supervisor. After three months, they should be re-labeled, and complete records of preparation, calibration, and re-labeling must be maintained. If any abnormalities such as cloudiness, sedimentation, or discoloration occur during storage, immediate action should be taken.
â–³ Preparation Quality Requirements
For standard solutions that do not require calibration, analytically pure substances can be used. The water used in preparation must meet the standards outlined in the pharmacopoeia. Calibration standards should not use reference substances. To prevent moisture absorption, the material should be dried to a constant weight before calibration. According to pharmacopoeia guidelines, perchloric acid titrants should be prepared at room temperature. If the temperature difference between usage and calibration exceeds 4°C or goes beyond 10°C, the solution should be recalibrated or adjusted accordingly.
Standard titrants must be prepared and calibrated by the QC supervisor. After three months, they should be re-labeled, and complete records of preparation, calibration, and re-labeling must be maintained. If any abnormalities such as cloudiness, sedimentation, or discoloration occur during storage, immediate action should be taken.
â–³ Preparation Quality Requirements
For standard solutions that do not require calibration, analytically pure substances can be used. The water used in preparation must meet the standards outlined in the pharmacopoeia. Calibration standards should not use reference substances. To prevent moisture absorption, the material should be dried to a constant weight before calibration. According to pharmacopoeia guidelines, perchloric acid titrants should be prepared at room temperature. If the temperature difference between usage and calibration exceeds 4°C or goes beyond 10°C, the solution should be recalibrated or adjusted accordingly.
 â–³ Titration Calibration and Labeling
Before calibration, the titration solution must be shaken and left to stand for three days. The calibration method should follow the procedures specified in the pharmacopoeia. Typically, one person performs the calibration, while a second person conducts a parallel test. At least three parallel determinations should be carried out. The relative deviation of the results should not exceed 0.1%.
The relative deviation between the first and second test results should not exceed 0.15%. If this limit is exceeded, but the recalibration meets the requirements, the average of the two results should be used as the final titration value.
Qualified titrants and standard solutions must be clearly labeled with the product name, concentration, preparation date, calibration date, temperature, calibrator, preparer, and expiration date. Titrants should be relabeled periodically, and their shelf life is generally three months. Once expired, they should not be used.
Titrants and standard solutions should be stored according to the guidelines provided in the Chinese Pharmacopoeia. Solutions that are sensitive to light should be stored in brown bottles. The containers should be protected from moisture and contamination, and should be managed by designated personnel. Any standard solution or titrant that has passed its expiration date must not be issued by the custodian or used by the end-user.
â–³ Titration Calibration and Labeling
Before calibration, the titration solution must be shaken and left to stand for three days. The calibration method should follow the procedures specified in the pharmacopoeia. Typically, one person performs the calibration, while a second person conducts a parallel test. At least three parallel determinations should be carried out. The relative deviation of the results should not exceed 0.1%.
The relative deviation between the first and second test results should not exceed 0.15%. If this limit is exceeded, but the recalibration meets the requirements, the average of the two results should be used as the final titration value.
Qualified titrants and standard solutions must be clearly labeled with the product name, concentration, preparation date, calibration date, temperature, calibrator, preparer, and expiration date. Titrants should be relabeled periodically, and their shelf life is generally three months. Once expired, they should not be used.
Titrants and standard solutions should be stored according to the guidelines provided in the Chinese Pharmacopoeia. Solutions that are sensitive to light should be stored in brown bottles. The containers should be protected from moisture and contamination, and should be managed by designated personnel. Any standard solution or titrant that has passed its expiration date must not be issued by the custodian or used by the end-user.

"Puzzle 3D Eraser Bulk,Puzzle 3D Eraser,Building Blocks Eraser,Toppers Cute Erasers "
Yiwu Shengshang Stationery Co., Ltd. , https://www.ss-stationery.com